Por Carlos Huerta-Aguilar y Pandiyan Thangarasu
Changes in the modern world have forced minimization, reuse and search of alternatives to traditional semiconductors. Rare earths for example, are scarce and expensive, nevertheless, electronic and chemical industry counts on them to maintain its production.
As an alternative, Carlos Alberto Huerta-Aguilar, researcher of the School of Engineering and Sciences at Campus Puebla is turning its interests towards iron oxides: they are found far and wide in the rust of metallic surfaces, as an impurity in urban tap water and in metal slags at construction sites1. However, specialty iron oxides have amazing properties despite its simple manufacturing. Magnetite, for example, is present in most magnetic devices and is used DNA purification, in manufacturing of high specialty components in informatics, optics, and instrumental medicine. Magnetite belongs to a group of oxides called ferrites, and as of 2021, the market shows sustained growth and an estimated size of USD 130.8 billion for 20262. The price of simple iron oxides as low as 5 USD/lb for Fe2O3 and ~90 USD/lb for Fe(OH)2. Yet, blends of ferrites with metals are much more expensive: ~338 USD/lb for iron ferrite (Fe2+Fe2O4) and 5,500 USD/lb for yttrium-iron oxide (Y3Fe5O12).
Even though all ferrites have similar structure, small changes in composition have tremendous effects 3: Magnetite has outstanding paramagnetic properties, zinc-cobalt ferrites are active under solar light and cerium and nickel ferrites are used in cancer tissue identification4-6. Mixture of ferrites with small amounts of rare earths can even outperform traditional semiconductors as gallium arsenide or silicon carbide. These blends diminish the supplies of bulk precursors and its robustness allows reutilization achieving a semicircular life cycle.
The simplicity of nanomaterials and its production
Simplicity boosts implementation and the simpler the material is produced, the higher the chance for large-scale application. Nanomaterials are regarded as very complex and exotic but actual engineering allows precise control 7. Today, nanoengineered compounds and are industrially produced and for magnetite nanoparticles, there is an annual growth around 10% and by 2026 it is estimated to reach USD 90 million8.
Researchers at Tecnologico de Monterrey, campus Puebla have produced ferrites that mixed with Cerium (CeFe) and Ruthenium (RuFe) are activated when irradiated with solar light. Additionally, they implemented synthetic processes that avoid toxic reagents and organic solvents as part of the Green Chemistry Initiative 20229. To produce the ferrites, a high pressure and temperature method known as hydrothermal is utilized. It consists on the mixture of metallic salts in water with pressurized cooking that result in materials that are recoverable using electromagnets due its magnetic behavior 10.
Understanding and modeling nanomaterials: Experimental and computational chemical engineering
Ferrites can be easily produced, but their properties need to be understood; therefore, a joint experimental-theoretical modelling was developed. The obtained information is used as feedback in subsequent manufacturing cycles in a tactic known as smart synthesis: In the first step, hydrothermal preparation takes place and Electronic Microscopy analyses unveil composition, size and shape: Our research group can continuously produce particles below 100 nm (one red blood cell is 8,000 nm across) and a homogenous composition which confirms the reliability of manufacturing process.
In a second stage, researchers created a computational model with a quantum approximation known as Density Functional Theory (DFT) using experimental information 11. Due complexity of the nanomaterials, high computational resources are necessary, and the UNAM-Tec de Monterrey partnership allowed the researchers the access to UNAM’s Miztli supercomputer. Obtained data is finally presented as a hybrid theoretical-experimental model where performance of the ferrites is predicted and explained at atomic micro scale even before it’s production.

Towards real applications in environmental sciences: Degradation of pollutants
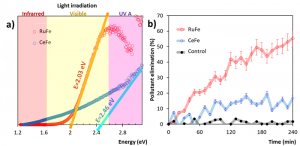
Modelling is useful but not enough for modern industry. To test the real performance of the prepared ferrites, our group tested the elimination of Congo Red, a common dye in textile. First, it is necessary to know if the materials can be activated by sunlight irradiation, and a technique called Diffuse Reflectance Spectra (DRS) is utilized. Indeed, RuFe and CeFe iron oxides are activated when light above 400 nm is present this corresponds to visible light found in regular sunlight.
Secondly, elimination of pollutants is tested: After 4 hours of contact between the dye and ferrites under direct sunlight, more than 50% of the contaminant was eliminated using RuFe oxides but only 20% with CeFe nanomaterials. This method of pollutants elimination is known as photocatalysis and its main advantage is that the final products are biodegradable residues or even nontoxic CO2 and water.
Heading towards green cycles and resource minimization with nanobiotechnology
This research is performed at Laboratorio de Energias Renovables in Campus Puebla. For the near future, our group is focusing on scaling up production and optimization of sunlight-harvesting materials in order to develop commercial applications for this technology. In the last year, three research papers were published; results were presented in American Chemical Society National Meeting at Atlanta, GA, USA and International Material Research Congress at Cancun, Q Roo, MX.
Authors
Carlos Alberto Huerta Aguilar ([email protected]): He got PhD in Environmental Engineering and currently has SNI 1. He is part of GIEE in Energy and Climate change.
Pandiyan Thangarasu ([email protected]): Researcher at Faculty of Chemistry, UNAM. Currently the Editor of the Journal of Environmental protection (JEP) and has SNI 3 category.
References
- Journal of Magnetism and Magnetic Materials 519, 167163 (2021).
- Lucintel, Report No. 1019302, 2021.
- Heliyon 5 (1), e01151 (2019).
- Environmental Science and Pollution Research 29 (5), 6698-6709 (2022).
- Sol. Energy Mater. Sol. Cells 219, 110786 (2021).
- Journal of Alloys and Compounds 829, 154533 (2020).
- Coatings 10 (3) (2020).
- Journal of Molecular Liquids 328, 115375 (2021).
- Chem. Eng. J. 381, 122596 (2020).
- A Zeitschrift für Physikalische Chemie 234 (4), 719-776 (2020).


















